A group of law firms leading the mass litigation on the LFIT Cobalt Chromium V40 Femoral Heads has announced a confidential settlement of hundreds of consolidated cases for the recalled metal hip parts.
Stryker has reached a settlement with patients who had revision surgery to replace the faulty components manufactured by Howmedica Osteonics Corp. The cases are part of multidistrict litigation in Massachusetts and New Jersey, which consolidated 125 and 140 cases in each state, respectively. Lawsuits from both jurisdictions are included in the private resolution.
Stryker LFIT Cobalt Chromium V40 Femoral Heads led to pain, loss of mobility, inflammation, instability, and tissue and muscle damage from corrosion of the metal femoral head. The serious reactions are similar to issues with other previously recalled Stryker hip implants, which released dangerous metal debris into the hip joint after metal-on-metal rubbing.
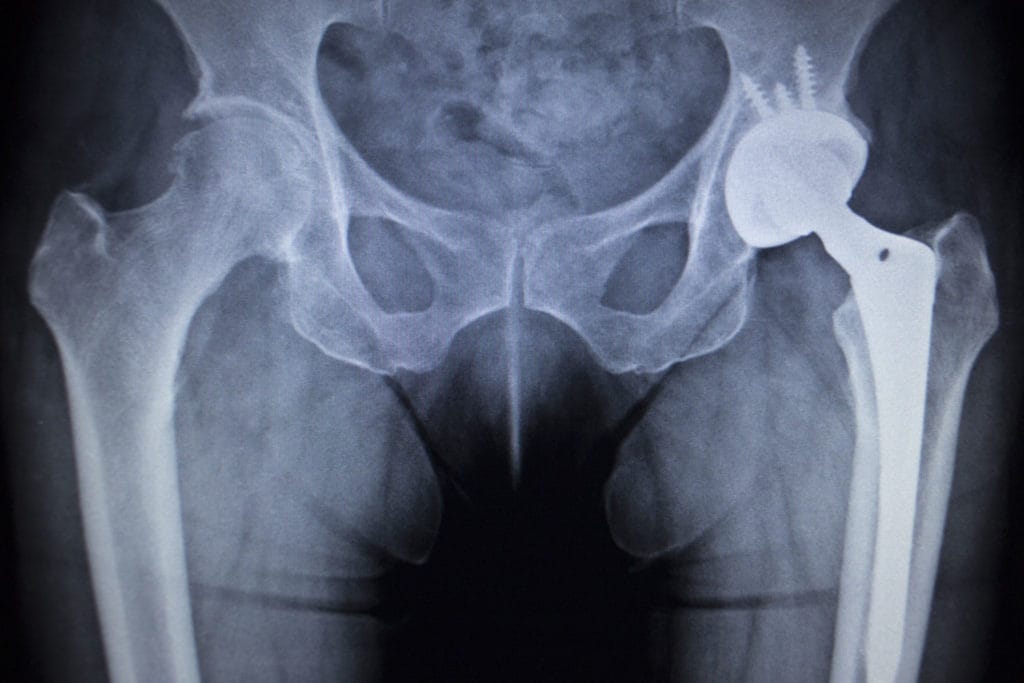
The affected Stryker hip replacements include the LFIT Cobalt Chromium V40 Femoral Head component, Stryker Rejuvenate and Stryker ABG II Modular Neck Stem. Plaintiffs in these cases reported both pain and suffering from the original Stryker implant, and also inconvenience and recovery related to the revision surgery.







